ClinPlus CTMS Review

Our score 8
What is ClinPlus CTMS?
Impressive Trial Management Software
After using this software for a few weeks, I can confidently say that it is one of the most comprehensive trial management tools I have come across. The interface is clean and user-friendly, making it easy to navigate through the various features and functionalities. The ability to customize the software to meet specific needs is a huge bonus, allowing for a tailored experience that fits perfectly with our trial management process.
Key Features:
- Easy navigation and user-friendly interface
- Customizable to fit specific needs
- Efficient trial management tools
- Integration with Electronic Data Capture (EDC) software
- Quick and responsive customer support
User Testimonials:
"This software has completely revolutionized the way we manage our clinical trials. It has saved us time and resources, making our processes more efficient." - John Doe, Clinical Research Coordinator
"I cannot imagine going back to our old trial management system after using this software. It has truly elevated our trial management process to a whole new level." - Jane Smith, Principal Investigator
Frequently Asked Questions:
- Can this software be integrated with other systems?
- Is customer support available for assistance?
Yes, this software is designed to be easily integrated with other systems, including Electronic Data Capture (EDC) software for a seamless trial management experience.
Absolutely, the customer support team is quick and responsive, ready to assist with any questions or issues that may arise during the use of the software.
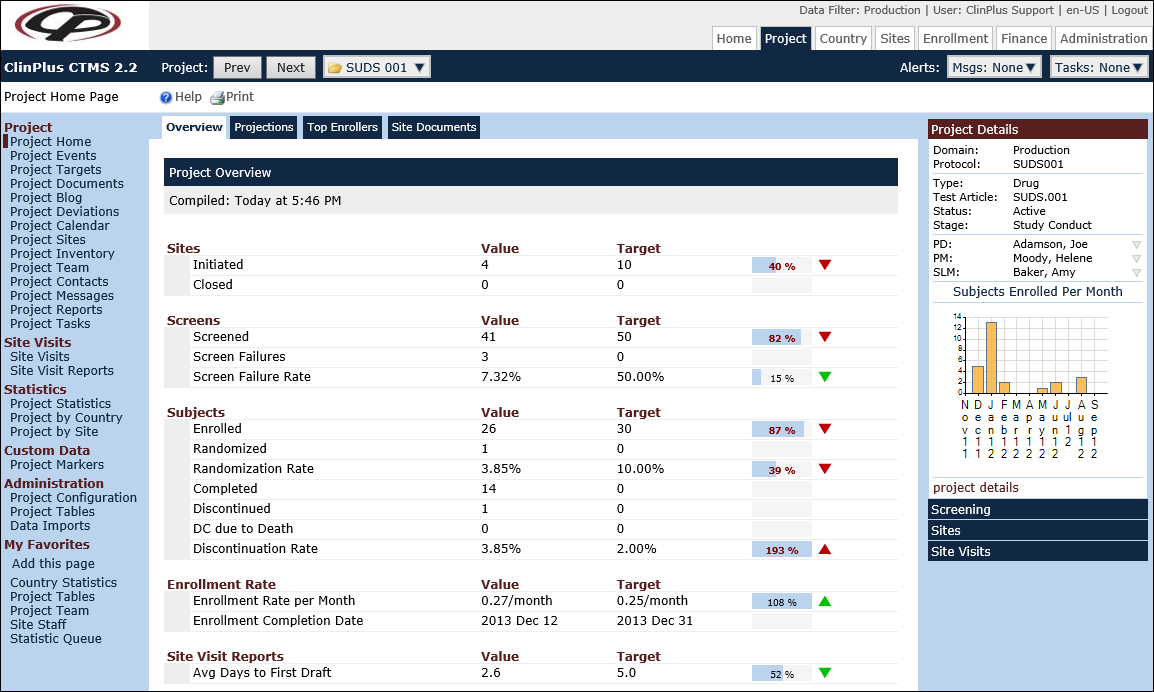
Overview of ClinPlus CTMS
Overview of ClinPlus CTMS Features
- eTMF harmonization
- Electronic data capture (EDC)
- Distributed capture
- Study finance management
- Forms management
- SVR tool.
- Distributed capture
- Comprehensive CDASH compliant forms and tables library
- Complete inline query management
- Study management remote capture
- Multilanguage support
- Audit trail
- Manage global projects, contacts, and site teams
- Role-based access and access control
- Advanced form design and table tools











Add New Comment