Castor EDC Review
Castor EDC
Life Sciences Software

Our score 9.2
What is Castor EDC?
Review of a Powerful and Cutting-Edge Software for the Life Sciences Industry
When it comes to software solutions for the Life Sciences industry, there is one product that truly stands out from the crowd. With its comprehensive and user-friendly features, this software has revolutionized the way data is captured and managed in clinical trials. With its Electronic Data Capture (EDC) capabilities and Clinical Trial Management Software functionality, it is no wonder that users are raving about it.
Unparalleled Features
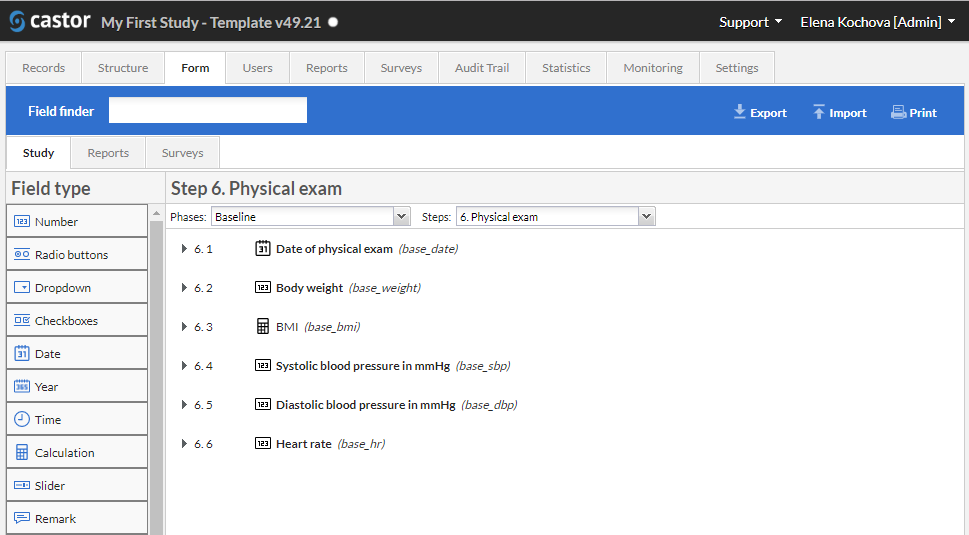
- Efficient Data Collection: This software offers a seamless data capture experience, allowing users to easily collect and input data in a highly organized manner. The intuitive interface ensures that users can navigate through the system effortlessly, saving time and reducing errors.
- Data Validation: One of the standout features of this software is its robust data validation capabilities. It automatically checks for inconsistencies, discrepancies, and missing data, helping users maintain the quality and integrity of their data sets.
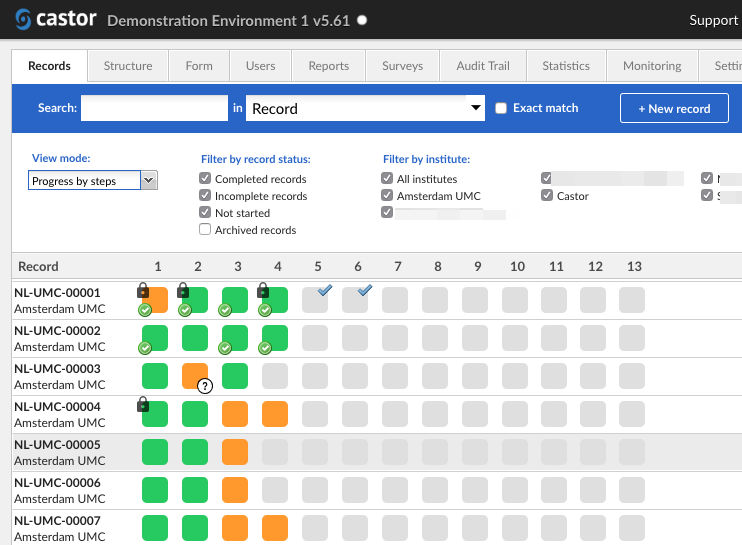
- Real-time Monitoring: With this software, users can monitor their clinical trials in real-time, providing them with valuable insights and actionable data. This feature greatly enhances the efficiency and effectiveness of trial management, enabling users to make informed decisions promptly.
- Customizable Reports: Generating detailed reports has never been easier. This software offers a wide range of customizable reporting options, allowing users to present their data in a visually appealing and meaningful way. From graphs and charts to tables and summaries, the possibilities are endless.
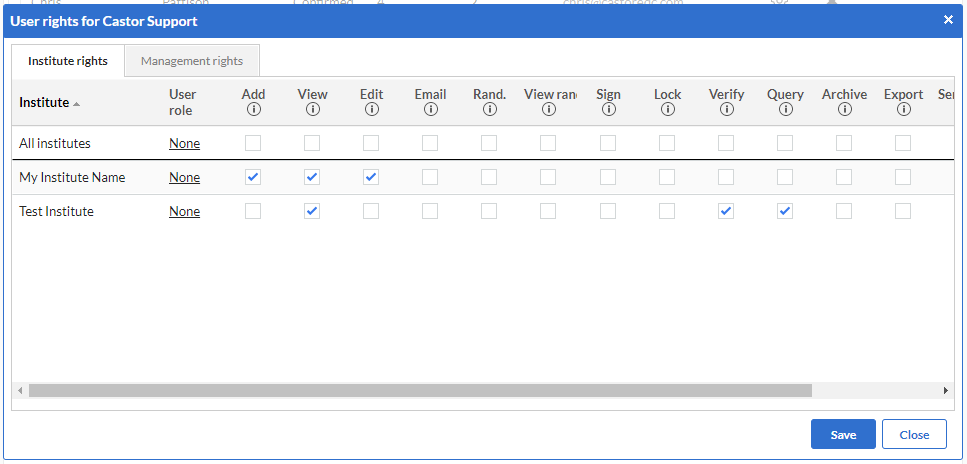
- Secure and Compliant: This software prioritizes data security and compliance with industry regulations. It employs state-of-the-art encryption techniques to safeguard sensitive data, ensuring that it remains confidential and protected at all times.
User Feedback
"This software has truly transformed the way we conduct clinical trials. Its user-friendly interface and powerful features have greatly streamlined our data collection and management processes. We have experienced significant time and cost savings since implementing this software." - Sarah S., Research Coordinator
"The real-time monitoring feature has been a game-changer for us. It allows us to track the progress of our trials and quickly identify any potential issues or trends. This has greatly improved our decision-making process and ultimately led to more successful trials." - John M., Clinical Project Manager
Key Features:
- Efficient data collection with an intuitive interface
- Robust data validation capabilities
- Real-time monitoring for actionable insights
- Customizable reporting options
- High level of data security and compliance
Frequently Asked Questions:
- Is training provided for using the software? Yes, comprehensive training and support are offered to facilitate a smooth onboarding process.
- Can the software be integrated with other systems? Absolutely, this software is designed to seamlessly integrate with existing systems, ensuring compatibility and data flow.
- Can I access the software from anywhere? Yes, this software is a cloud-based solution, enabling users to access their data and manage trials from anywhere with an internet connection.
Overview of Castor EDC
Seller :
Castor EDC
HQ Location :
Amsterdam, Netherlands
Year founded :
2011
Language supported :
English
Dutch
Devices Supported :
Windows
Android
iPhone/iPad
Mac
Web-based
Deployment :
Cloud Hosted
Customer Types :
Small Business
Large Enterprises
Medium Business
Pricing Model :
Annual Subscription
Quote-based
Free
Support :
Email
Phone
Live Support
Training
Overview of Castor EDC Features
- Two-Factor Authentication
- Source Data Verification
- Advanced Form Builder
- Automation Engine
- User Management
- Monitoring
- Data Capture
- Data Import & Export
- Randomization
- Data Protection
- Study Statistics & Overview
- Patient Surveys
Gallery
Videos
Page last modified
Share :
suggestVideo











Add New Comment