Clinical Conductor CTMS Review

Our score 8.8
What is Clinical Conductor CTMS?
Review: Clinical Conductor CTMS - Taking the Hassle out of Clinical Trial Management
As a professional in the field of clinical research, I am constantly on the lookout for software solutions that can simplify and streamline the complex process of managing clinical trials. Recently, I had the opportunity to use and test a promising solution that has garnered quite a reputation in the industry. Let me share my experience with this robust Clinical Trial Management Software.
Upon my initial interaction with this application, I was immediately impressed by its sleek and intuitive user interface. The layout is thoughtfully designed, allowing for easy navigation and quick access to the various features. Armed with a comprehensive suite of tools, this software provides all the capabilities necessary to efficiently oversee every aspect of a clinical trial.
Highlights:
- Powerful Study Management: With this software, managing multiple clinical studies becomes a breeze. From start to finish, it provides a range of features to facilitate study setup, site management, patient recruitment, and data collection. The ability to easily monitor study progress and track key milestones is invaluable.
- Seamless Collaboration: This software truly understands the importance of team collaboration in the clinical research process. It offers robust tools for sharing documents, assigning tasks, and facilitating real-time communication among team members. This collaborative environment fosters increased efficiency and promotes effective teamwork.
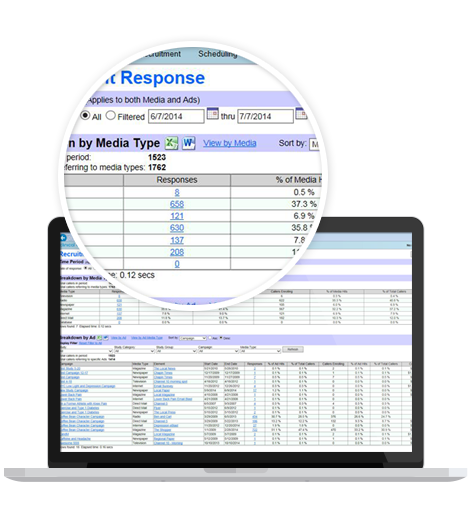
- Data Management and Analysis: The software's data management capabilities are top-notch. It enables users to efficiently capture, store, and organize critical patient and study data. Moreover, with its powerful reporting and analytics functionality, it empowers researchers to gain valuable insights and make data-driven decisions.
- Regulatory Compliance: Compliance with regulatory standards can be a daunting task, but this software ensures that clinical trials adhere to the necessary guidelines. It facilitates the creation of regulatory documentation, streamlines the adverse event reporting process, and provides audit trails for enhanced transparency.
Now, let's take a moment to hear what other users have to say about this software:
"Clinical Conductor CTMS has revolutionized the way we manage our clinical trials. It has not only saved us time and resources, but also significantly improved our efficiency in study execution." - John, Clinical Research Coordinator
"This software is a game-changer for our organization. Its user-friendly interface and comprehensive feature set make it an indispensable tool in our clinical trial management process." - Sarah, Clinical Trial Manager
Key Features:
- Intuitive and user-friendly interface
- Comprehensive study management capabilities
- Efficient data capture and organization
- Powerful reporting and analytics
- Real-time collaboration and communication
- Regulatory compliance support
Frequently Asked Questions:
- Q: Is this software suitable for both small and large-scale clinical trials?
- A: Absolutely! The software is highly scalable and can effectively cater to the needs of clinical trials of any size.
- Q: Can I access the software remotely?
- A: Yes, the software is cloud-based, allowing users to access it from anywhere with an internet connection.
- Q: Does the software support integration with other systems?
- A: Yes, the software provides integration capabilities with other commonly used clinical research applications, enhancing overall efficiency.
- Q: Can I rely on the software for regulatory compliance?
- A: Absolutely! The software is designed to help users adhere to regulatory standards, ensuring compliance throughout the clinical trial process.
In conclusion, Clinical Conductor CTMS has exceeded my expectations as a powerful and user-friendly Clinical Trial Management Software. Its comprehensive feature set, ease of use, and seamless collaboration make it an essential tool for any organization involved in clinical research. I highly recommend giving it a try!
Overview of Clinical Conductor CTMS
Overview of Clinical Conductor CTMS Features
- Patient Visit Scheduling
- Study Setup & Templates
- Appointment Checklists
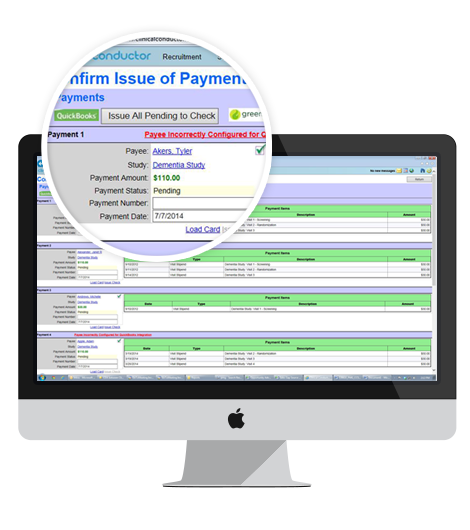
- Patient Payment System
- Web Recruitment
- Contact Management
- Reporting
- Budget Negotiation
- Document Sharing
- Budgets & Payments Tracking
- Patient Database
- Document Storage & Tracking













Add New Comment